From science to therapy
Working collaboratively, we are harnessing cutting-edge methodologies to realize the full potential of our proprietary MAT platform
Mitochondrial Augmentation
Based on the proven ability of isolated mitochondria to enter cells, we have researched and developed our science of mitochondrial augmentation into a therapeutic platform: Mitochondrial Augmentation Technology (MAT), an investigational, proprietary mitochondrial transplantation approach aimed to treat the root cause of diseases caused by mitochondrial dysfunction.
We have demonstrated the ability to enrich multiple cell types with mitochondria, ranging from hematopoietic cells such as hematopoietic stem cells, B cells, CARTs, and TILs, to non-hematopoietic cells such as MSCs. In its first clinical uses, Mitochondrial Augmentation Technology enriches the patient’s hematopoietic stem and progenitor cells with healthy mitochondria isolated from an allogeneic or syngeneic source.
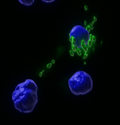
We have demonstrated, using a variety of visual, molecular, and biochemical methodologies that syngeneic or allogeneic mitochondria can enter cells in a dose dependent manner to enrich mitochondrial content and rescue cellular function.
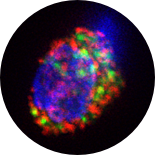
GFP labeled mitochondria
in CD34+ cells
Enhancing Patient-Derived Hematopoietic
Stem & Progenitor Cells with Healthy Mitochondria
In the clinical setting, we currently perform MAT on autologous Hematopoietic Stem and Progenitor Cells (HSPCs). These are the progenitors of every blood and immune cell in the body - both the innate and adaptive immune systems, cells of both myeloid and lymphoid lineages. As stem cells, they are long-lived; as hematopoietic cells, their daughter cells circulate systemically and reach every organ system in the body, a key factor when addressing multi-systemic diseases.
First and foremost, enriching HSPCs with healthy mitochondria impacts their ability to function and differentiate into active myeloid and lymphoid cells. Therefore, mitochondrial augmentation of HSPCs is expected to improve a variety of hematopoietic and immune phenotypes.
Interestingly, scientific literature supports the use of HSPCs to treat not only hematopoietic, but also non-hematopoietic disorders. For example, bone marrow transplant (BMT) is used to alleviate brain dysfunction in diseases such as Hurler’s Syndrome. In addition, allogeneic HSCT (HSC transplantation) using bone marrow or umbilical cord blood as the source for HSPCs has been shown to alleviate hematopoietic, but also brain, kidney and liver dysfunction of mitochondrial disease in preclinical and clinical studies. Together, these provide scientific rationale for possible non-hematopoietic, systemic effects of augmented HSPCs.
Due to the associated morbidity and mortality, allogenic HSCT is not generally indicated for patients with mitochondrial dysfunction. The power of MAT is that it can be performed using autologous HSCTs, affording the benefit of a HSPC-based therapy without the associated allogeneic risks.
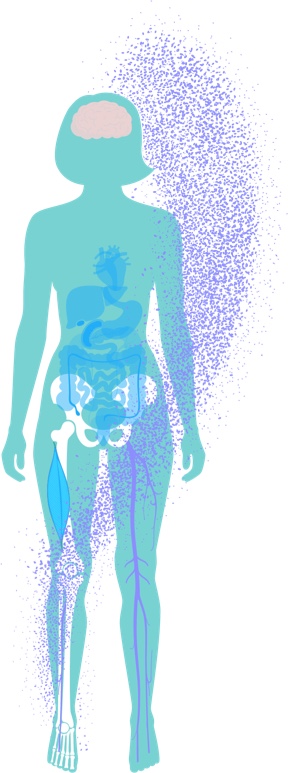
Systemic Effects of MAT
After HSPCs are enriched with healthy mitochondria, they are re-infused back to the patient, where they circulate through the bloodstream and can home to hematopoietic and non-hematopoietic tissue.
Mitochondrial transfer has been demonstrated to occur in vitro and in vivo via a number of biological mechanisms, including extracellular vesicles and tunnelling nanotubes. Using novel mouse models, we are studying the persistence of mitochondrial transfer in hematopoietic and non-hematopoietic tissues at different time points after MAT.
Initial preclinical results indicate continuous transfer of allogeneic mitochondria to recipient hematopoietic cells months after a single infusion of augmented HSPCs. Importantly, it was previously shown that mitochondrial transfer occurs to higher extents in preclinical models of injury, suggesting higher mitochondrial transfer to cells in a state of metabolic stress due to mitochondrial dysfunction.
We are harnessing established preclinical cell and animal models to help us understand the systemic effect of MAT on hematopoietic and non-hematopoietic tissues, including assessment of MAT’s effect on gene expression and metabolic pathways. In addition, we are studying the effect of MAT on the immune system and defining immunomodulatory roles of MAT as part of the systemic mode of action.