Mitochondrial Augmentation Technology (MAT)
Young and healthy mitochondria isolated from healthy donors are transplanted in patients suffering from mitochondrial dysfunction

Lead product MNV-201
Manufacturing and cryopreservation of a qualified mitochondria product derived from healthy donor placenta
Patient’s Hematopoietic Stem and Progenitor Cells (HSPCs) are mobilized and collected from the blood by apheresis
Autologous CD34+ HSPCs are selected from the apheresis product
Selected cells are enriched with healthy mitochondria in a 24 hour process
The mitochondrially augmented cells are qualified and infused to patient’s bloodstream
* MAT is an investigational therapy that is undergoing clinical study to verify its safety and effectiveness.
Additional information can be found on ClinicalTrials.gov.
Placenta
Our first generation products enabled proof of concept in human clinical use. However, this personalized approach is low throughput and only enables treatment of patients with non-inherited disorders for whom a maternal mitochondrial donation is available.
Enter placenta-derived allogeneic mitochondria, our second generation mitochondria. The placenta is a young, ephemeral tissue, which can be harvested in a sterile environment during a scheduled C-section. Each placenta yields hundreds of vials of highly active placenta-derived mitochondria, which can be fully qualified with a wide array of quality assays confirming activity and safety. The placenta donor does not need to be related to the recipient of treatment. This enables a scalable, off-the-shelf solution, with which any cell type can be enriched.
Mitochondrial Diseases
Primary Mitochondrial Diseases (PMDs) are a heterogeneous group of disorders caused by mutations or deletions in nuclear or mitochondrial DNA (mtDNA), displaying a wide range of severity and phenotypes. These complex, multi-systemic and rare group of diseases occur as a result of mitochondrial dysfunction.
A subset of PMDs is a group of diseases caused by single-large scale mitochondrial DNA deletions (SLSMDS) such as Pearson Syndrome and Kearn-Sayre Syndrome (KSS), both are life-threatening systemic multi organ diseases. PMDs caused by mutations in the mtDNA include Leigh Syndrome, MELAS and MERRF. Currently, there are no approved disease modifying drugs for PMDs and patients are treated with supportive care only.
Chronic diseases, as well as age-related diseases are known to be associated with mitochondrial dysfunction. Amassing evidence has demonstrated the involvement of mitochondrial dysfunction in a variety of neurodegenerative disorders (Alzheimer, Parkinson, ALS), Cardiovascular diseases, Chronic Kidney Disease, Diabetes, Myelodysplastic Syndrome and more.
Myelodysplastic Syndrome (MDS):
MDS are defined by ineffective hematopoiesis resulting in blood cytopenia, and clonal instability with a risk of evolution to Acute Myeloid Leukemia (AML). Patients with MDS collectively have a high symptom burden and are also at risk of death from complications of cytopenia and AML. The goals of therapy for patients with MDS are to improve cytopenia, reduce disease-associated symptoms and the risk of disease progression and death, thereby improving both quality of life and lifespan. The median age at diagnosis of MDS is ~70 years, but surprisingly some of the Pearson Syndrome patients develop MDS in a much higher prevalence relative to the disease population. About 15% of the MDS patients will present with Sideroblastic Anemia, the most common symptom in Pearson Syndrome.
Minovia developed novel blood biomarkers to measure mitochondrial health, and was able to demonstrate for the first time that MDS is actually an age-related mitochondrial disease. As such, Minovia proposes MNV-201 for the treatment of low-risk MDS.
Aging is presented with many of the symptoms observed in mitochondrial diseases. Moreover, mitochondrial dysfunction is an underlying cause of many age-related disease, including metabolic syndromes, neurodegenerative diseases, cardiovascular diseases and cancer. Aging is also associated with hematopoietic stem cells exhaustion and impaired immune system which results in poor resistance to infections. Minovia's MAT platform can be applied to determine if a patient suffers from mitochondrial dysfunction and rescue the dysfunction by transplanting healthy, young and functional mitochondria.
Beyond HSPCs: Mitochondrial augmentation of additional cell types.
While Minovia's primary focus has been augmentation of HSPCs, we are exploring the potential benefit of augmentation of other mitochondrially exhausted cell types. For example, to treat hematopoietic and solid tumors, CART and TILs have been used in the past decade. However, one major limitation is the durability of effect due to metabolic exhaustion of cells. We are actively exploring the potential benefit MAT may provide additional cell therapies under development.
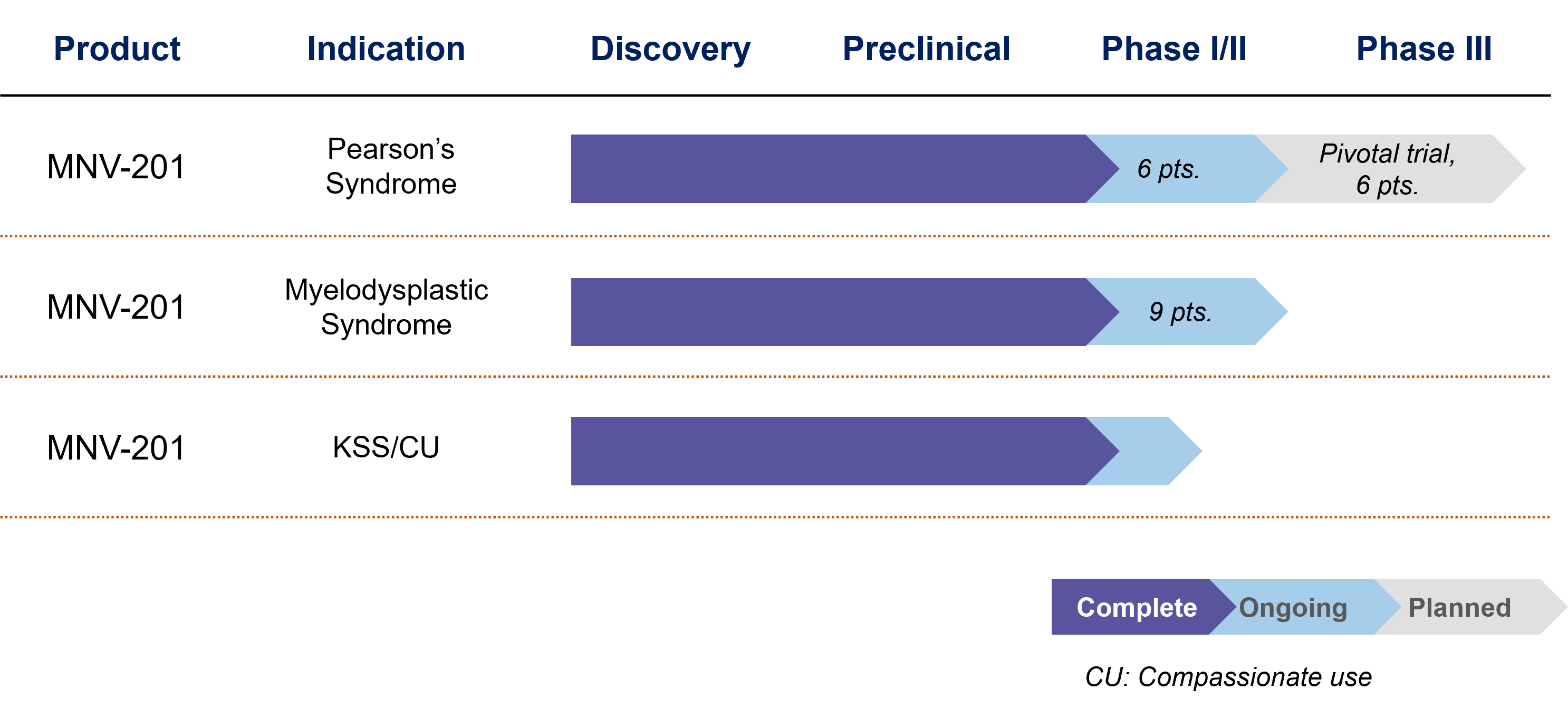
Clinical Development
Minovia is currently evaluating the MAT platform to treat both rare genetic mitochondrial diseases, as well as age-related mitochondrial dysfunction disorders.
First generation (Syngeneic maternal mitochondria, MNV-101): In our first clinical study of MAT, Pearson Syndrome patients were treated with autologous HSPCs enriched with healthy, functional mitochondria derived from maternal while blood cells. This personalized therapy was developed with the potential to treat only non-inherited mitochondrial disorders, and has shown a favorable safety and efficacy profile in a phase I/II clinical trial for Pearson Syndrome and in compassionate use cases for KSS and Leigh Syndrome. The compassionate use cases of single-large mitochondrial DNA deletions was published in Science Translational Medicine.
Second generation (Allogeneic mitochondria, MNV-201): Our second generation lead investigational product is currently in human studies for Pearson Syndrome and KSS, and in pre-clinical studies for low-risk Myelodysplastic Syndrome (MDS). NV-201 has the potential to treat all Primary Mitochondrial Disease (PMDs), as well as secondary disorders associated with mitochondrial dysfunction.

Our Pipeline
MNV-201 is currently in clinical trials for Pearson Syndrome – a primary mitochondrial disease and for Low-Risk Myelodysplastic Syndrome (MDS)
Q & A
Why did we select Pearson Syndrome as our lead program focus for our first generation product?
Pearson Syndrome is an ultra-rare, fatal disease. Usually the main symptom at diagnosis is associated with the bone marrow, therefore HSPCs are the right modality to treat this disease. It is not inherited, allowing the mother to be a donor of healthy mitochondria to her child.
How do we address inherited mitochondrial diseases specifically in situations when the mother cannot be the mitochondria donor?
For patients affected with an inherited mitochondrial disease, an allogeneic source of healthy mitochondria will be used for MAT. For this purpose, we have chosen the placenta, a young, healthy, ephemeral organ rich in mitochondria. Feasibility and safety studies are conducted to test matching requirements of allogeneic mitochondria carrying different haplotypes to personalize the therapy.
How many times do patients require MAT?
The current clinical trial protocols assess a single treatment. We continue to follow up on all patients, monitoring specific biomarkers, as well as the patient’s well-being and overall clinical condition to make informed decisions about a potential need for redosing. Ongoing preclinical studies aim to assess durability of affect.
Will placenta matching be required for augmentation with the second generation product?
No. To date, none of the studies performed at Minovia suggest a requirement for matching between the donor mitochondria and recipient cells. Actually, the fact that mitochondria come from an unmatched donor is useful, in that it may allow tracking of persistence of the donor mitochondria over time.
Might use of allogeneic mitochondria cause an immune response?
Just as no mitochondrial matching is performed when an organ transplant is provided, or when a blood or platelet transfusion is performed, it is highly unlikely that an immune response will occur. No immune responses have been evident in any preclinical studies, even when mitochondria and recipient cells were unmatched. However, as a safety precaution, responses will be carefully monitored in any human studies.
I/my loved one has a disorder you currently do not treat in a clinical trial. Where can I get information on whether you may treat this in the future?
We constantly strive to develop MAT to reach as many patients as possible, and are acutely aware of the unmet needs of many patients with diseases associated with mitochondrial dysfunction. Please do not hesitate to reach out to us; we will save your details and reach out as soon as possible.
Do you need to harvest mitochondria from my placenta?
No, the new generation of augmented cells utilizes a mitochondrial bank harvested from healthy donated placentae. Each patient treated will receive cells augmented from this preexisting bank.
I understand you have a treatment center in Israel; is this currently the only site where MAT treatment is possible?
While in the future we plan to open sites around the globe, currently treatment is only possible at Sheba Medical Center, the hospital performing our clinical trials.
What is the connection between Pearson Syndrome and Myelodysplastic Syndrome?
Both PS and MDS are disorders in which the stem cells of the blood system do not function properly, leading to disease. Scientific literature supports mitochondrial dysfunction as being involved in the hematopoeitic stem cells of both disorders. Interestingly, a subset of MDS patients present with ring sideroblasts, one of the defining symptoms of bone marrow of Pearson patients. Intriguingly, a larger than expected proportion of Pearson Syndrome patients have been diagnosed with MDS, supporting a causal link between mitochondrial dysfunction and MDS. Therefore, it is possible that the same treatment may alleviate symptoms involved in both disorders.